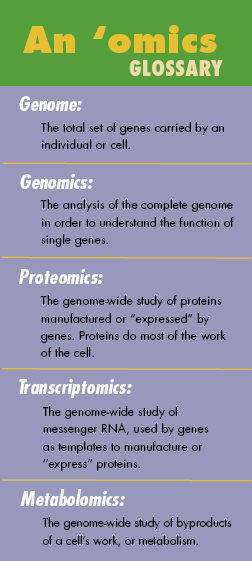
For generations, scientists have worked to understand the cell down to its smallest components. Today researchers in the fields of genomics, proteomics, transcriptomics and metabolomics seek to understand the cell as a whole, in all its complexity.
Elbert Branscomb, associate director of the Biology and Biotechnology Research Program at Lawrence
Livermore National Laboratory, likens cells to small cities.
"Think of a cell as the whole city of Chicago, at rush hour, busily making another Chicago," says Branscomb,
who is also a member of the UC Davis Integrated Cancer Research Program.
The mind spins at the immensity of the challenge: Each human cell has at its disposal about 30,000 genes,
the sum of the human genome. Together these genes are capable of producing, via a manufacturing process
known as transcription, perhaps 100,000 different proteins. It's the proteins that carry out the cell's
work, or metabolism. How many byproducts of metabolism there are — the metabolites — is unknown;
a common estimate is 3,000.
For cancer research, the implications are tremendous. "We are moving towards an understanding of
cancer that will enable us to tailor-make the optimal treatment for each person," says Hsing-Jien
Kung, deputy director of the UC Davis Cancer
Center and director of its basic science program. "This university has made a tremendous, multidisciplinary
commitment to this revolution, and we are leading the way into the future."
A dramatic convergence of new developments fueled the 'omics revolution. Foremost was the mapping of
the human genome, followed closely by gene-array analysis. This powerful new technology allows scientists
to sequence thousands of genes at a time in a matter of days. Once-formidable tasks, like pinpointing
the genetic differences between two populations — with and without a certain cancer, for example
— today are commonplace.
At the same time, old tools have become super-efficient. Less than a generation ago, mass spectrometry
could screen for 20 metabolites in a milliliter of urine or blood. The newest machines can screen for
thousands in a sample as small as 1/1,000th of a milliliter.
But Branscomb says the biggest factor driving the 'omics revolution is a new mindset. "The most important
lesson we've learned from the genomics revolution is that it is often as easy to measure many things as
to measure one thing," he says. "Now that we have the capacity to do mass analysis, we are approaching,
systematically, problems that were unthinkable just three to four years ago."
Like using genomics, proteomics, transcriptomics and metabolomics to develop a unique fingerprint for
each individual. With this information, scientists look forward to the day they will be able to predict
an individual's propensity to develop cancer or a cancer patient's response to a certain therapy. Doing
so will make cancer a controllable, if not a preventable, disease.