Fomina Laboratory
Membrane Trafficking
Insertion and internalization of proteins into the plasma membrane (PM) is mechanistically linked with cell growth and differentiation. In addition, rapid insertion or internalization of channels, receptors, or transporters into the PM may be triggered by extracellular stimuli. Despite their importance, the mechanisms regulating insertion or removal of channels or receptors in T lymphocytes are poorly understood. We introduced the use of the styryl dye FM 1-43 to investigate real-time membrane trafficking in living T lymphocytes by fluorescence microscopy, followed by a snapshot of endocytic compartments with transmitted electron microscopy, to correlate the fluorescence measurements with the ultrastructure of endocytic organelles (Pub Link). FM1-43 is an amphipathic molecule that intercalates spontaneously into but does not penetrate through the biological membranes. Within the lipid environment, FM1-43 exhibits 50 to 100-fold increased fluorescence intensity than the dye in aqueous solution. FM1-43 can be internalized by vesicular endocytosis and an increase in intracellular fluorescence can therefore, be used as an index of endocytosis. Moreover, intense illumination of FM1-43 in fixed specimens in the presence of oxygen and diaminobenzidine catalyzes formation of an insoluble osmiophilic reaction product that can be visualized by electron microscopy.
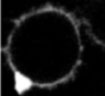
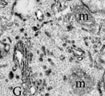
When FM1-43 was introduced into the cell culture media, it rapidly stained the PM of both resting and activated T cells as a result of dye partitioning into the lipid bilayer. Incubation of resting or activated T cells in the presence of FM1-43 resulted in fast internalization of the dye from the PM into the endocytic compartments by constitutive endocytosis (Movie 1). Although ultrastructural characteristics of early endocytic compartments were similar in resting and activated T cells (Figure 1), comparison of the time courses of FM1-43 accumulation in living cells revealed that the rate of constitutive membrane trafficking was ~ 10 fold higher in activated T cells compared to resting.
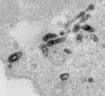
Our study also demonstrated dramatic differences between structure of the late endocytic compartments in resting and activated T lymphocytes (Figure 2). In resting T cells, FM1-43 was found in lysosome-like vacuoles that lacked internal membranes, whereas activated T cells readily accumulated the dye within multivesicular bodies (MVB). We found that limiting membrane of MVB can fuse with the PM and release the internal vesicles, termed exosomes, into the extracellular space (Figure 3). This pathway may allow certain proteins to avoid degradation and initiate signal transduction in neighboring cells.
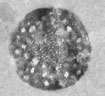
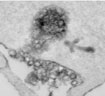
Because elevation in cytosolic Ca2+ concentration ([Ca2+]i) plays a pivotal role in many T cell functions, we investigated the effects of Ca2+ on membrane trafficking in T cells (Pub Link). We have found that elevation in [Ca2+]i accelerated the rate of FM1-43 accumulation in human and Jurkat T lymphocytes. At the ultrastructural level, we observed that FM1-43 penetrated the lumen of peripheral endoplasmic reticulum (ER) followed by the dye spreading into the nuclear envelope and golgi apparatus (Figure 4). Areas of close apposition of ER and PM membranes are evident in unstimulated cells and remained intact in cells with FM1-43 present in the ER lumen (Figure 5). These observations suggest that FM1-43 transfer into the ER lumen could occur via ER-PM contact sites and that perturbation of Ca2+ homeostasis may create continuity between the PM and the ER, resulting in direct membrane exchange between those organelles.
Our present research focuses on studying the mechanisms regulating exocytosis in T cells. Using capacitance detection technique and total internal reflection fluorescent microscopy (TIR-FM), we are recording the exocytotic events in real time from individual cells. We are exploring the role of cytosolic Ca2+, phosphoinositides, cytoskeletal elements, and fusion proteins in regulation of exocytosis of membrane proteins (e.g. ion channels and receptors) and/or diffusible factors (e.g. interleukins and/or exosomes). The results of this research may reveal new fundamental mechanisms regulating intracellular communications in T cells and identify novel pharmacological targets for management of autoimmune disorders.




 Make a donation using our secure online system.
Make a donation using our secure online system.