Resident Program - Case of the Month
May 2018 - Presented by Dr. Dongguang Wei (Mentored by Dr. Kristin Olson)
Discussion
What is Composite Intestinal Adenoma-Microcarcinoid?
In contrast to neuroendocrine tumors of the colon, which are grossly evident neoplasms with neuroendocrine differentiation, the Composite Intestinal Adenoma-Microcarcinoid (CIAM) is an uncommon colorectal lesion predominantly consisting of a conventional adenomatous component with microscopic foci of neuroendocrine-type cells interwoven with the adenoma (1-4).
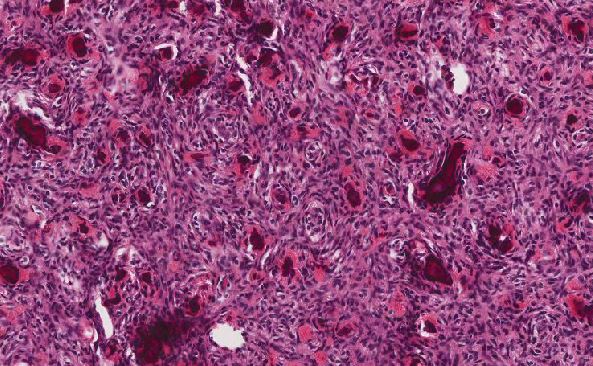
Histologic Features of CIAM
The adenomatous components of this lesion are classically conventional adenomas (tubular adenoma or tubulovillous adenoma) of variable size and degree of dysplasia, with some demonstrating mucosal prolapse or desmoplastic-like fibrosis (2). The “microcarcinoid” components represent only a small portion of the polyp. No sharp demarcation between the glandular component and the microcarcinoid component is seen. Architecturally, the microcarcinoid component is arranged in nests or cords near the base of the adenomatous crypts, and tends to occur in the basal portion of lamina propria (1-3). This component ranges from 1-20 mm in size, and is usually present multifocally within the individual adenoma, with or without connections to the neighboring adenoma. “Drop-off” of neuroendocrine cells from the base of the adenomatous glands may be seen, and the surrounding stroma may mimic desmoplasia (3). In polyps with mucosal prolapse, the clusters of microcarcinoid cells may be enveloped by a fibromuscular proliferation worrisome for an infiltrating adenocarcinoma. Rarely, the microcarcinoid cells breach the muscularis mucosae, which can also raise concerns for invasion. Cytologically, the microcarcinoid cells are small and bland with plentiful to moderate pale, granular cytoplasm. The nuclei are monotonous and round with finely stippled chromatin and inconspicuous nucleoli. Concerning features such as pleomorphism, hyperchromasia, mitotic figures, and necrosis are usually absent.
The histological origin of the CIAM is not clear. Hypothesized origins include a common precursor cell with divergent differentiation to adenomatous and microcarcinoid components (6); a collision of two distinct, separately arising entities; or metaplasia secondary to chronic injury of the adjoining adenoma.
Immunohistochemical Profile of the Microcarcinoid Component of CIAM
The microcarcinoid component is immunoreactive for synaptophysin, with variable reactivity to chromogranin A and CD56 (1-4). The microcarcinoid also tends to display strong and diffuse nuclear β-catenin reactivity (1). Some microcarcinoids may demonstrate squamoid differentiation with variable reactivity to p63 and/or CK5/6 (1). The low mitotic rate observed in the H&E stain is confirmed by Ki-67 nuclear proliferative index staining <2%. The microcarcinoid should demonstrate wild-type p53 immunostaining (1).
Clinical Outcome
Microcarcinoids are well-recognized in the setting of type A gastritis (5). CIAMs, in contrast, are relatively rare, and their clinicopathologic characteristics remain to be addressed. Our current understanding of this entity suggests that CIAM has a favorable clinical outcome; thus, surgery is not routinely considered if the lesion has been completely excised endoscopically. However, resection is still the treatment of choice in cases of submucosal invasion and increased proliferative activity.
Differential diagnosis/diagnostic pitfalls
Adenocarcinoma: The CIAM is a little recognized mimic of invasive adenocarcinoma. Microcarcinoid features that can simulate adenocarcinoma include the presence of small angulated nests of neuroendocrine cells or even single cell permeation, the drop-off neuroendocrine cells from the base of the adenomatous glands, an overlying high-grade dysplastic glandular component, edematous lamina propria simulating desmoplasia, and/or prolapse-associated fibrosis simulating infiltration. The absence of hyperchromasia, pleomorphism, mitotic figures, and necrosis are more indicative of a benign process. Furthermore, strong nuclear reactivity for β-catenin, non-reactivity for p53, and a minimal Ki-67 proliferation index are all helpful in differentiating CIAM from adenocarcinoma.
Classic neuroendocrine tumors (NET): In contrary to classic gastrointestinal neuroendocrine tumors, CIAMs are most frequently found in the colon, and the proximal colon in particular (2). The expansile nodular or organoid growth pattern characteristic of classic neuroendocrine tumors is absent in CIAMs. The nests/clusters of the NE component found in CIAM weave within the glandular components in the basal lamina propria, unlike the classic gastrointestinal NETs centered in the submucosa. Additionally, the NE component in CIAM is usually asymptomatic and only found during microscopic examination, while colonic NETs are frequently symptomatic and identified endoscopically (7).
Take-Home Messages
- CIAM is an uncommon colorectal lesion predominantly consisting of a conventional adenomatous component with microscopic foci of neuroendocrine-type cells interwoven with the adenoma.
- CIAM may demonstrate squamous differentiation.
- CIAM can mimic infiltrative adenocarcinoma with its edematous stroma that simulates desmoplasia, or its prolapse-associated fibromuscular proliferation that simulates invasion.
- Key features of microcarcinoids:
- There is no sharp demarcation between the glandular and microcarcinoid components.
- Nuclear pleomorphism, hyperchromasia and mitotic activity is usually absent in microcarcinoids.
- Microcarcinoids are generally positive for synaptophysin with variable reactivity to chromogranin A and CD56, strong/diffuse nuclear β-catenin reactivity, low proliferative index (Ki-67 < 2%), and no reactivity to p53.
References
- Salaria SN, Abu Alfa AK, Alsaigh NY, Montgomery E, Arnold CA. Composite intestinal adenoma-microcarcinoid clues to diagnosing an under-recognised mimic of invasive adenocarcinoma. J Clin Pathol 2013; 66 (4):302-306.
- Kim MJ, Lee EJ, Kim DS, Lee DH, Youk EG, Kim HJ. Composite intestinal adenoma-microcarcinoid in the colon and rectum: a case series and historical review. Diagn Pathol. 2017;12(1):78.
- Lin J, Goldblum JR, Bennett AE, Bronner MP, Liu X. Composite intestinal adenoma-microcarcinoid. Am J Surg Pathol. 2012;36(2):292-5.
- Pulitzer M1, Xu R, Suriawinata AA, Waye JD, Harpaz N. Microcarcinoids in large intestinal adenomas. Am J Surg Pathol. 2006;30(12):1531-6.
- Reinecke P, Borchard F. Pattern of gastric endocrine cells in microcarcinoidosis–an immunohistochemical study of 14 gastric biopsies. Virchows Arch. 1996;428:237–41.
- Vortmeyer AOLI, Merino MJ, Wang CY, et al. Concordance of genetic alterations in poorly differentiated colorectal neuroendocrine carcinomas and associated adenocarcinomas. J Natl Cancer Inst. 1997;89:1448–1453.
- Bosman FT, Carneiro F, Hruban RH, Theise ND. World Health Organization classification of Tumours of the digestive system. 4th ed. Lyon: IARC Press; 2010. p. 160–5.

 Meet our Residency Program Director
Meet our Residency Program Director