Residency Program - Case of the Month
October 2010 Final Diagnosis - Presented by Sarah Barnhard, M.D.
Answer:
Chondrosarcoma, Grade 1
Histologic description
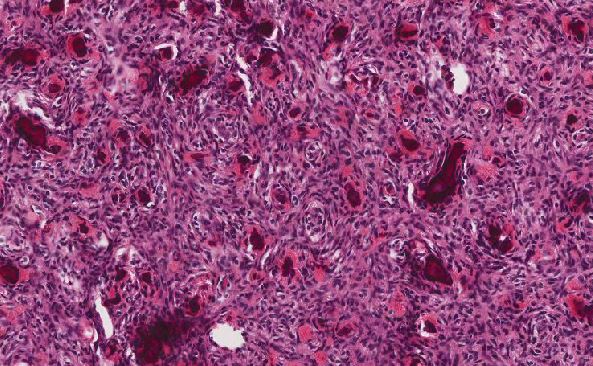
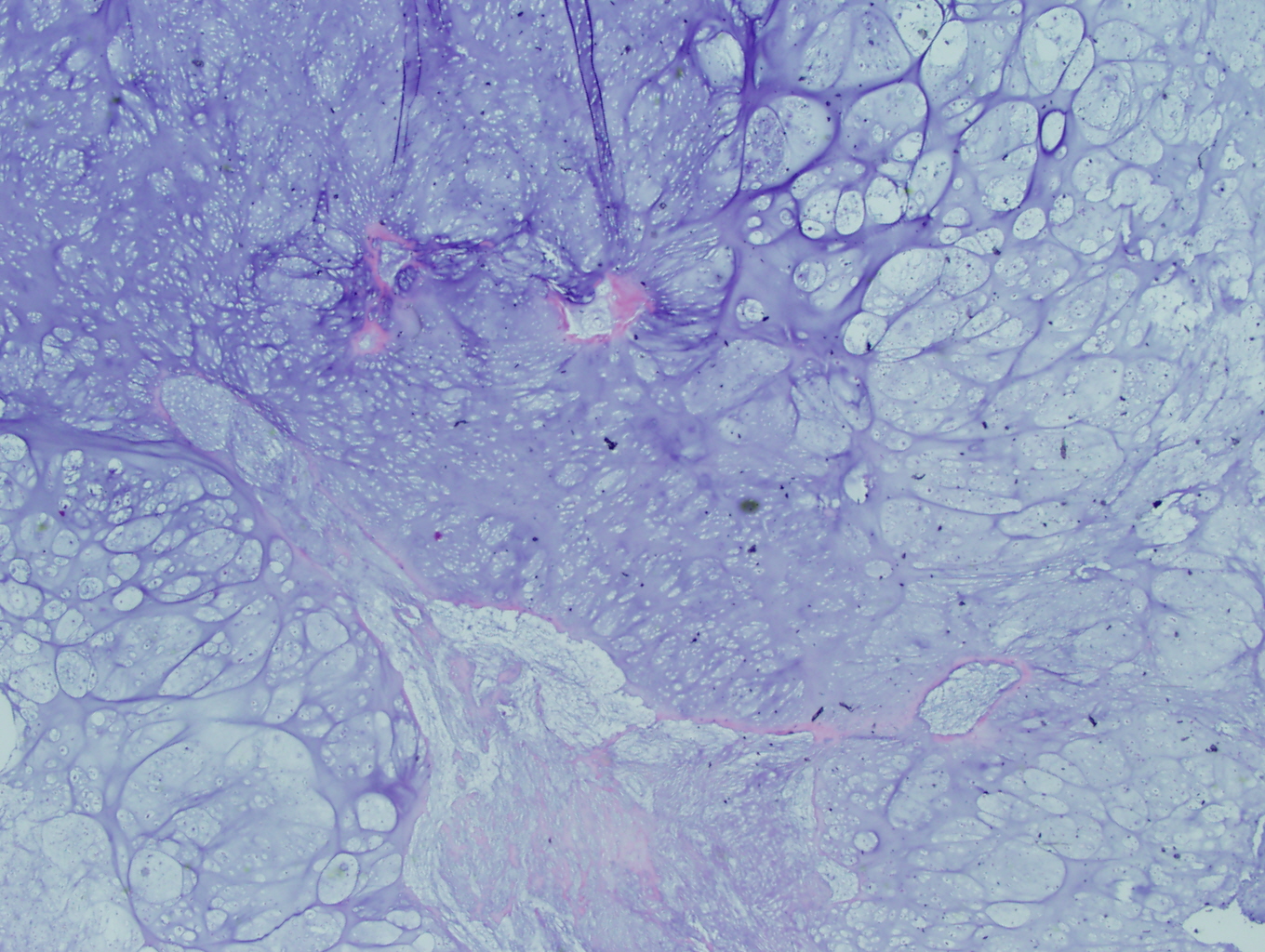
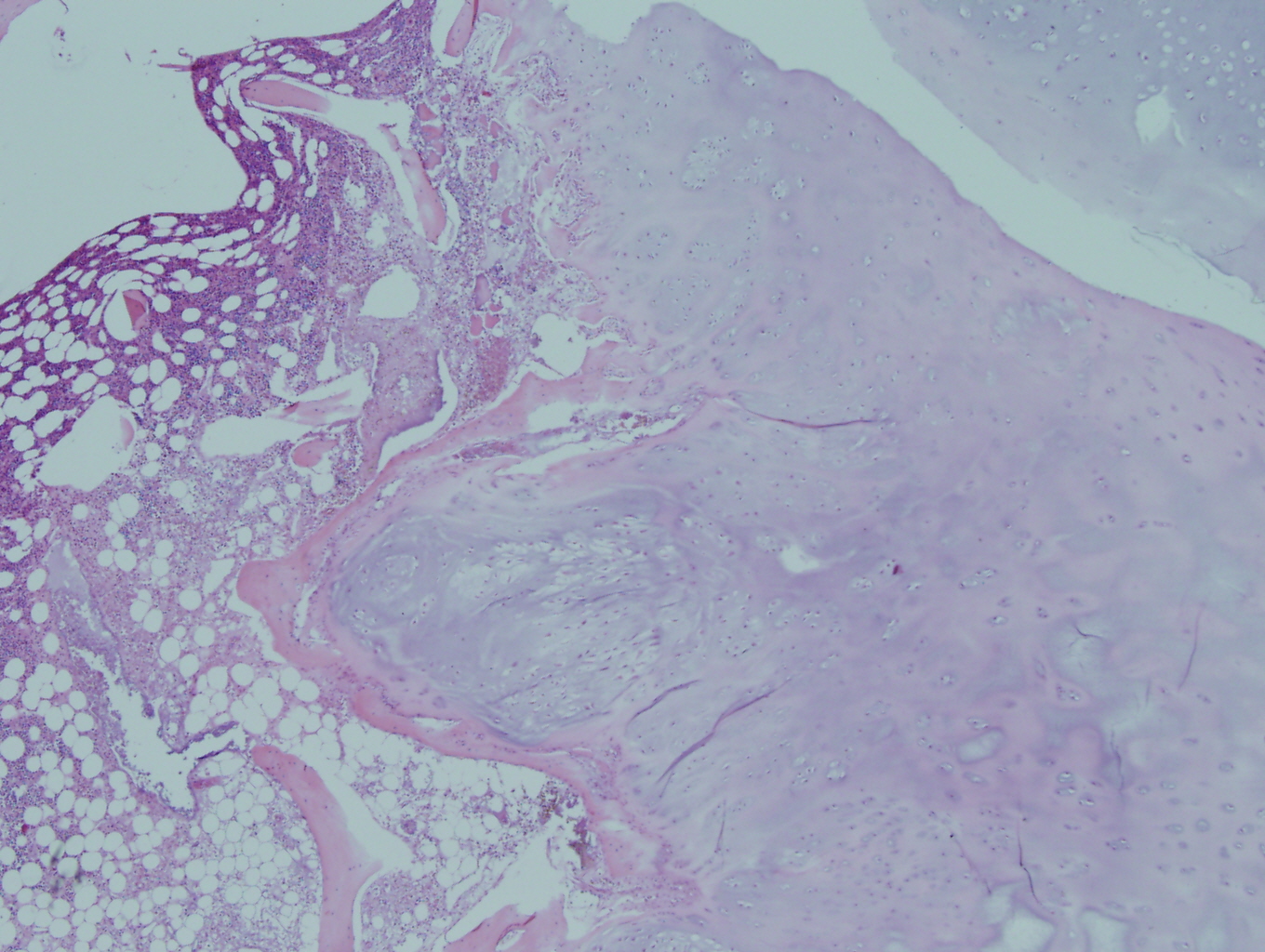
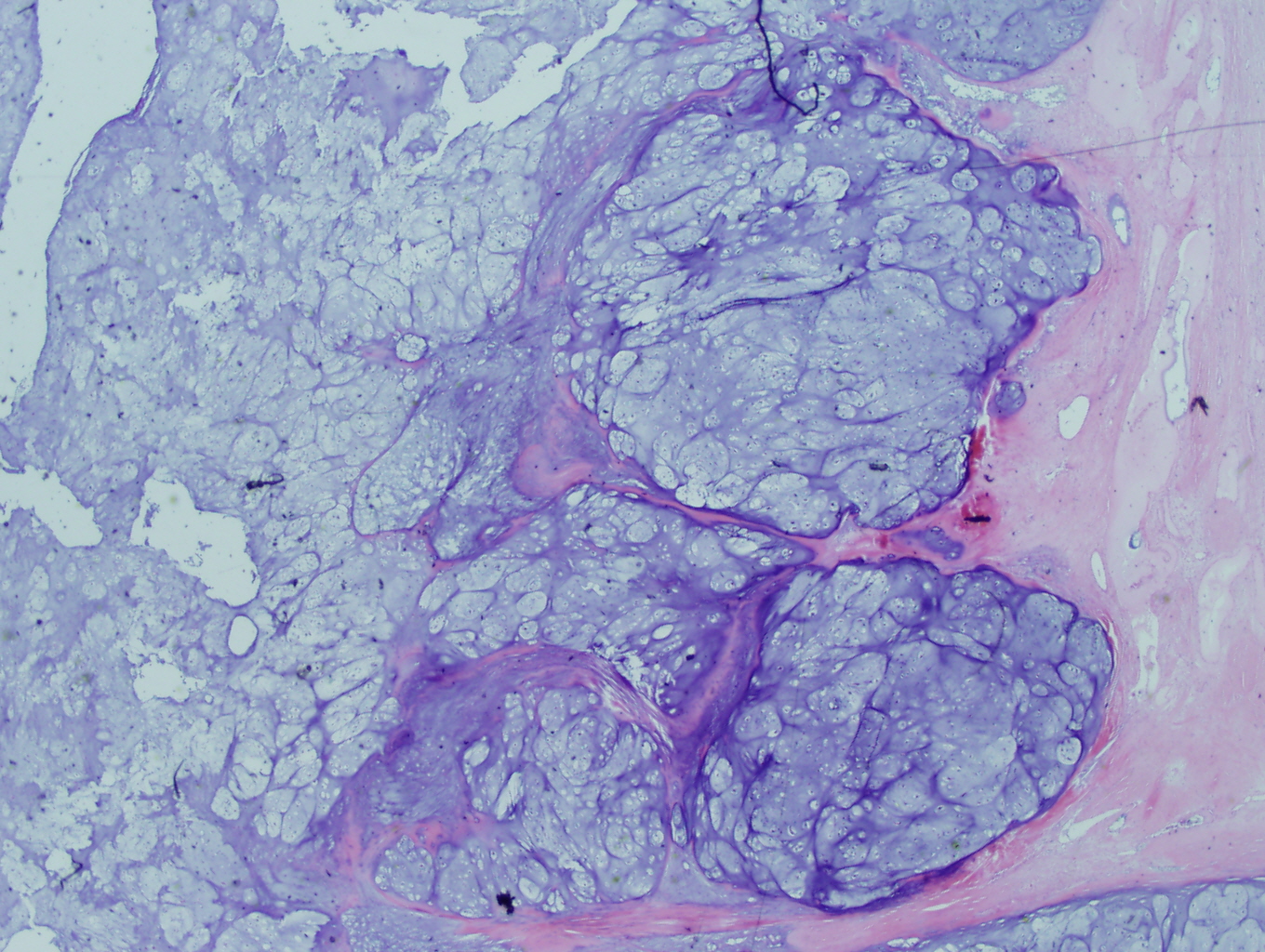
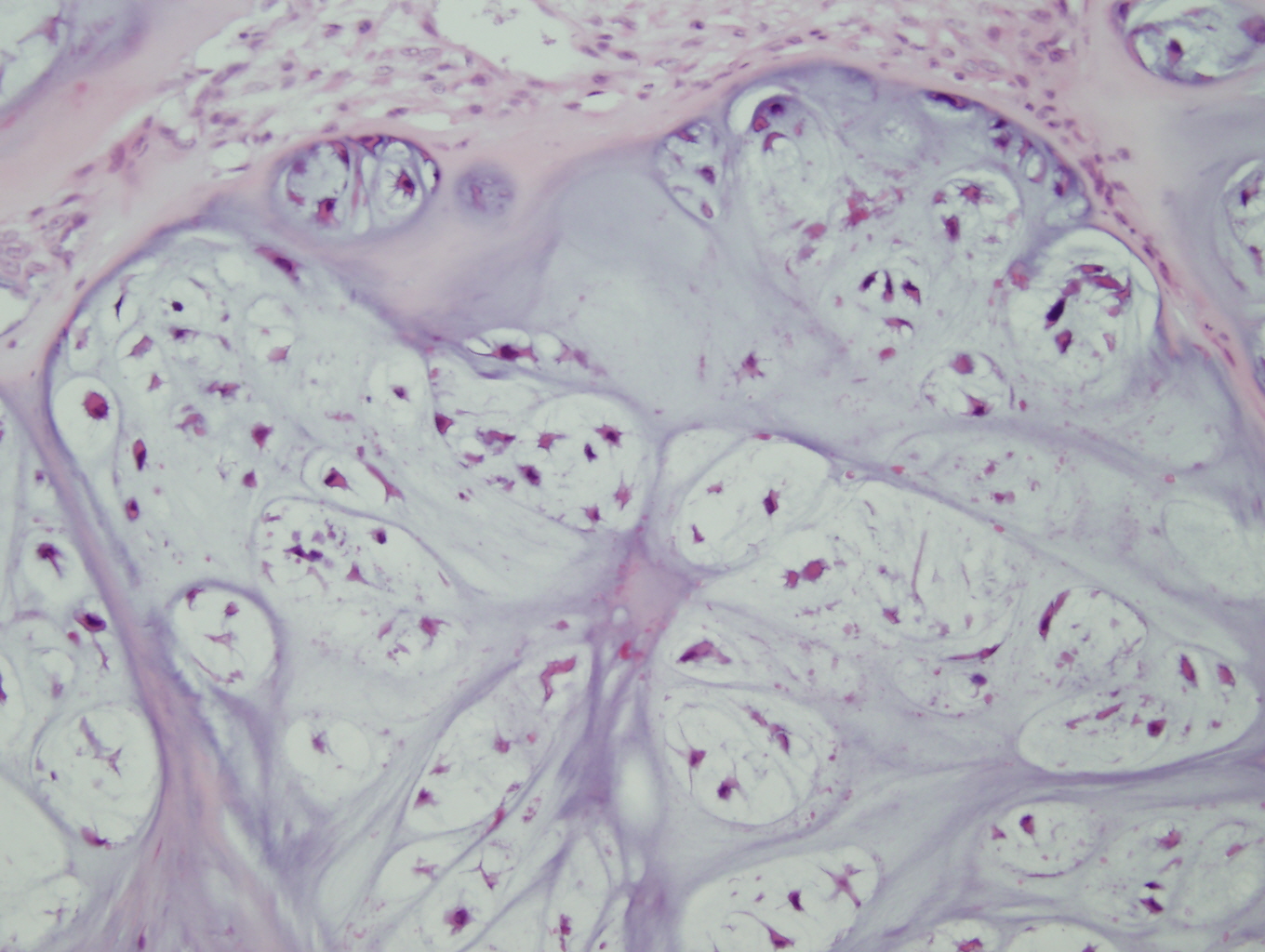
Microscopic examination revealed a hyaline and myxoid cartilage tumor of low to moderate cellularity arranged in nodules and sheets (Figure 1). The tumor was seen to permeate the cancellous bone of the marrow, completely surrounding and encasing fragments of cancellous bone (Figure 2). Additionally, the tumor expanded into the adjacent tissues, forming nodules of tumor surrounded by bands of fibrosis (Figure 3). Mitotic figures were present in areas of new bone formation which had a reactive bone appearance. Tumor cells were located within lacunae and had small and pyknotic nuclei (Figures 4). Rare areas of necrosis were present. Areas of delicate lace-like osteoid and plump atypical osteoblasts characteristic of osteosarcoma were absent.
|
Figure 1: Low power view demonstrating the lobulated architecture with abundant cartilaginous matrix that is characteristic of grade 1 chondrosarcoma |
Figure 2: Low power view of the tumor permeating the cancellous bone of the marrow and encasing fragments of cancellous bone |
|
Figure 3: Higher power view of the tumor expanding into adjacent tissue |
Figure 4: High power view of tumor cells in lacunar spaces with small pyknotic nuclei and surrounded by an abundant cartilaginous matrix |
Discussion:
Chondrosarcoma is the second most common primary bone tumor, surpassed only by osteosarcoma, and is almost exclusively a tumor of adulthood. The peak incidence of chondrosarcoma occurs in the fourth to sixth decade of life, with males affected as equally as females (1). It has been reported to occur in children under the age of 17 in only 2% of cases (2).
In adults, chondrosarcoma occurs predominantly in the proximal femur with other common locations being the knee, shoulder, pelvis, and spine. In children, the most common location has been reported to be the proximal humerus, although the spine and the shoulder and pelvic girdles are common locations as well. Radiologic-clinical-pathologic correlation is of utmost importance in forming a diagnosis. Determining malignancy of cartilaginous cells based solely on histologic examination is often difficult as low-grade malignancies may display a paucity of hypercellular tissues and very few plump, atypical or bizarre nuclei (3). Radiographic imaging of central chondrosarcomas typically demonstrates a lobulated lesion centered in the femoral metaphysis. Plain film of centrally located lesions shows endosteal scalloping, periosteal thickening, and stippling with "ring and arc" calcifications. The stippling indicates a cartilaginous matrix. The presence of extensive destruction of the cortex is the radiologic feature that clearly separates a chondrosarcoma from a benign enchondroma. Because of the weakened cortex, the patient usually complains of dull, localized pain which is not experienced with a benign mass lesion. The criteria used for diagnosis of chondrosarcoma in children include features similar to the criteria used for the same diagnosis in adults.
This malignant cartilage-producing mesenchymal neoplasm has several identifiable morphologic types which span a histologic grading scale of low to intermediate to high grade. Further, the aggressiveness of chondrosarcoma can be predicted by its histologic grade, which is based on three parameters: cellularity, degree of nuclear atypia, and mitotic activity. Conventional chondrosarcoma is subdivided by location in the bone: central, peripheral, or juxtacortical. The majority of conventional chondrosarcomas are primary lesions and are located centrally. A few (up to 15% in some reports) develop from the surface of the bone as the result of transformation within the cartilage cap of a pre-existing osteochondroma. These are termed secondary or peripheral chondrosarcomas by the WHO classification. The variant form of chondrosarcoma is divided into several histologic morphologies: clear cell, myxoid, mesenchymal, and dedifferentiated. The variant forms of chondrosarcoma, with the exception of the clear cell type, are typically grade 2 or grade 3 lesions.
Low-grade chondrosarcomas are resistant to radiation and chemotherapy. However, in general, the prognosis for a primary low-grade central chondrosarcoma is good, with a low rate of pulmonary metastasis if the primary lesion is widely resected.
Interestingly, the vast majority of childhood chondrosarcoma cases are observed as secondary lesions arising from osteochondromas in children with hereditary multiple exostoses (HME), a genetic disorder with autosomal dominant inheritance. Though our patient did not have the diagnosis of HME, there was a strong suggestion that his lesion originated in an osteochondroma given his age and the peripheral location of the lesion. The rate of malignant degeneration of osteochondromas is 1-5% and generally does not occur in patients prior to skeletal maturity. However, Dr. Schmale at the University of Washington with collaborators at Children’s Hospital of Seattle found two cases of chondrosarcomatous progression of an osteochondroma in skeletally immature patients, reflecting this unusual complication of MO. His team found that, clinically, the symptoms of malignant progression in adults had an insidious onset followed by a period of rapid growth of the tumor mass. With a lesion in a growing child, however, pain, swelling, and enlargement were non-specific symptoms. Their clinical course was much more insidious (2).
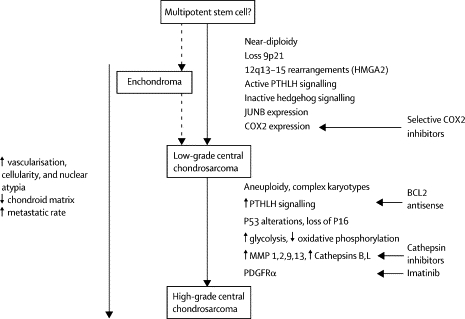
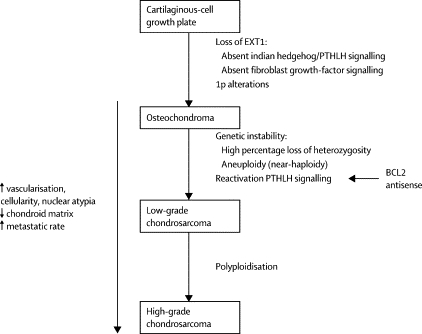
Also of interest is a paper published in 2005 in Lancet Oncology by Bovee et. al. proposing genetic models for both central and peripheral, that is primary and secondary, chondrosarcomas (figures are below). The models are based on the histology of the normal growth plate in which chondrocytes proliferate, differentiate, become hypertrophic, and eventually undergo apoptosis leading to longitudinal bone growth. Substances in the indian hedgehog (IHH)/parathyroid hormone like hormone (PTHLH) pathway have been shown to tightly regulate this process, and therefore research teams have drawn parallels between chondrocyte growth and differentiation in the normal growth plate and in tumors. For example, the EXT genes have been identified as causing multiple osteochondromas (5); these genes are also involved in signaling pathways in the normal epiphyseal growth plate.
Further, it has been found that cartilaginous tumors are nearly always found in bones arising from endochondral ossification. As the skeleton matures, most bones develop by the ossification of cartilage that originates from fetal mesenchyme (endochondral ossification). Areas of membranous ossification, that is areas where bone is formed directly from fetal mesenchyme, are rarely affected by cartilaginous tumors. Thus, there is an imperative to continue studying parallels of normal and neoplastic chondrocyte growth and differentiation.
References:
- Bertoni F, Bacchini P, and Hogendoorn PCW. Chondrosarcoma: World Health Organization classification of tumors. Pathology and genetics of tumors of soft tissue and bone. IARC Press, 2002;247-251.
- Evans, Harry MD. Prognostic Factors in Chondrosarcoma of Bone: A Clinicopathologic Analysis with Emphasis on Histologic Grading. Cancer 1997;40:818-831.
- Schmale G. Malignant Progression in Two Children with Multiple Osteochondromas. Sarcoma 2010 Online Publication 10.1155;417105.
- Bovee JV. Emerging Pathways in the Development of Chondrosarcoma of Bone and Implications for Targeted Treatment. Lancet Oncol 2005;6(8):599-607.
- AA Sandberg and JA Bridge, Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors: chondrosarcoma and other cartilaginous neoplasms, Cancer Genet Cytogenet 2003;143:1–31.
- Terek, RM Recent Advances in the Basic Science of Chondrosarcoma. Orthop Clin North Am. 2006;37(1):9-14.
- Hasegawa T, Seki K, and Yang P. Differentiation and proliferative activity in benign and malignant cartilage tumors of bone. Hum Pathol 1995;26:838.

 Meet our Residency Program Director
Meet our Residency Program Director